How Do You Think The Size Of An Atom Will Affect Its Ability To Attract Additional Electrons?
Electronegativity and Oxidation Number
Electronegativity is the tendency of an atom/molecule to attract electrons; oxidation number is an indicator of its bonding environment.
Learning Objectives
Use the rules for assigning oxidation numbers to atoms in compounds
Key Takeaways
Central Points
- An atom 's electronegativity is affected by both the element 's atomic number and its size.
- The college its electronegativity, the more an chemical element attracts electrons.
- The cantlet with higher electronegativity, typically a nonmetallic element, is assigned a negative oxidation number, while metal elements are typically assigned positive oxidation numbers.
Cardinal Terms
- electronegativity: A chemical property that describes the tendency of an atom to attract electrons (or electron density) toward itself.
- oxidation number: The hypothetical charge that an cantlet in a molecule/compound would have if all bonds were purely ionic. It indicates of the degree of oxidation of an atom in a chemical compound.
Electronegativity
Electronegativity is a property that describes the trend of an atom to attract electrons (or electron density) toward itself. An cantlet's electronegativity is affected by both its atomic number and the size of the atom. The higher its electronegativity, the more an chemical element attracts electrons. The reverse of electronegativity is electropositivity, which is a measure of an element's power to donate electrons.
Electronegativity is non straight measured, simply is instead calculated based on experimental measurements of other atomic or molecular properties. Several methods of calculation have been proposed, and although there may exist modest differences in the numerical values of the calculated electronegativity values, all methods testify the same periodic tendency among the elements.
Electronegativity, as information technology is usually calculated, is not strictly a property of an atom, but rather a belongings of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. Information technology is expected that the electronegativity of an element volition vary with its chemical environment, but it is usually considered to exist a transferable property; that is to say, similar values volition be valid in a variety of situations.
On the most basic level, electronegativity is determined past factors such as the nuclear charge and the number/location of other electrons present in the atomic shells. The nuclear charge is important because the more protons an atom has, the more "pull" it volition have on negative electrons. Where electrons are in space is a contributing gene because the more electrons an atom has, the farther from the nucleus the valence electrons will exist, and as a result they will feel less positive charge; this is due to their increased distance from the nucleus, and because the other electrons in the lower-energy core orbitals volition human activity to shield the valence electrons from the positively charged nucleus.
The nearly commonly used method of calculation for electronegativity was proposed by Linus Pauling. This method yields a dimensionless quantity, commonly referred to as the Pauling scale, with a range from 0.7 to iv. If nosotros look at the periodic table without the inert gases, electronegativity is greatest in the upper right and lowest at the bottom left.

Electronegativity of the elements: Electronegativity is highest at the tiptop right of the tabular array and lowest at the bottom left.
Hence, fluorine (F) is the about electronegative of the elements, while francium (Fr) is the least electronegative.
Oxidation Numbers
Information technology is mutual to consider a unmarried value of electronegativity to exist valid for nigh bonding situations a given atom can be in. While this arroyo has the reward of simplicity, it is clear that the electronegativity of an chemical element is non an changeless atomic property; rather, information technology can be thought of as depending on a quantity called 'the oxidation number' of the element.
One way to characterize atoms in a molecule and keep track of electrons is by assigning oxidation numbers. The oxidation number is the electric accuse an cantlet would have if the bonding electrons were assigned exclusively to the more electronegative atom, and it can identify which atom is oxidized and which is reduced in a chemical process. Half-dozen rules can be used when assigning oxidation numbers:
- The oxidation number of an element in its natural state (i.eastward., how information technology is found in nature) is null. For example, hydrogen in Hii, oxygen in Otwo, nitrogen in N2, carbon in diamond, etc., take oxidation numbers of zero.
- In ionic compounds, the ionic charge of an atom is its oxidation number.
- The sum of the oxidation numbers of all the atoms in an ion or molecule is equal to its net accuse.
- In compounds with nonmetals, the oxidation number of hydrogen is +ane. However, when hydrogen is bonded with a metal, its oxidation number reduces to -1 because the metallic is a more electropositive, or less electronegative, chemical element.
- Oxygen is assigned an oxidation number of -ii in virtually compounds. All the same, there are certain exceptions. In peroxides (Otwo 2-), such as hydrogen peroxide (H2Otwo), the oxidation number of oxygen is -ane. In oxygen difluoride (OFtwo), the oxidation number of oxygen is +2, while in dioxygen difluoride (O2F2), oxygen is assigned an oxidation number of +one because fluorine is the more electronegative element in these compounds, so it is assigned an oxidation number of -ane.
- The atom with higher electronegativity, typically a nonmetallic chemical element, is assigned a negative oxidation number, while the other atom, which is frequently merely not necessarily a metallic element, is given a positive oxidation number.
Bail Polarity
Molecular polarity is dependent on the presence of polar covalent bonds and the molecule's three-dimensional structure.
Learning Objectives
Apply cognition of bond polarity and molecular geometry to identify the dipole moment of molecules
Cardinal Takeaways
Cardinal Points
- When non-identical atoms are covalently bonded, the electron pair will exist attracted more than strongly to the atom that has the higher electronegativity. This results in a polar covalent bond.
- Polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment.
- A polar molecule acts equally an electric dipole that can interact with electric fields that are created artificially, or that arise from nearby ions or polar molecules.
- The dipole moment [latex]\mu[/latex] that corresponds to an private bond is given by the product of the quantity of charge, q, and the bond length r: [latex]\mu = qr[/latex].
Cardinal Terms
- Bond polarity: A covalent bond is polar if one atom is more electronegative than its bonding partner, resulting in a net dipole moment betwixt the ii atoms.
- dipole moment: A measure of the polarity of a covalent bond or of an unabridged molecule. It is the product of the charge on either pole of the dipole and the distance separating them.
- Molecular polarity: A molecule is polar if it has a net dipole moment, which depends on the existence of polar covalent bonds and the molecule's iii-dimensional structure or geometry.
Bond vs Molecular Polarity
Polarity refers to the separation of charge that creates permanent positive and negative 'electric poles.' This concept tin be practical in 2 contexts:
- Bail polarity: when atoms from different elements are covalently bonded, the shared pair of electrons volition be attracted more strongly to the atom with the higher electronegativity. As a result, the electrons volition not be shared as. Such bonds are said to exist 'polar' and possess partial ionic grapheme.
- Molecular polarity: when an entire molecule, which can be made out of several covalent bonds, has a net polarity, with one end having a higher concentration of negative charge and some other cease having a surplus of positive charge. A polar molecule acts as an electrical dipole which can interact with electric fields that are created artificially, or that arise from interactions with nearby ions or other polar molecules.
Dipole Moment
Dipoles are conventionally represented as arrows pointing in the direction of the negative terminate. The force of a dipole's interaction with an electric field is given by the electrical dipole moment of the bond or molecule. The dipole moment is calculated past evaluating the product of the magnitude of separated charge, q, and the bond length, r:
[latex]\mu = q r[/latex]
In SI units, q is expressed in coulombs and r in meters, so μ has the dimensions of [latex]C \cdot thou[/latex]. If two charges of magnitude +one and -1 are separated by a typical bond length of 100 pm, and so:
[latex]\mu = (1.6022 \times 10^{-19} C) \times (10^{-x} thousand) = 1.6 \times 10^{-29} C \cdot m = 4.viii D[/latex]
The Debye unit of measurement, D, is commonly used to express dipole moments.
Determining a Molecule's Dipole Moment
In molecules containing more one polar bond, the molecular dipole moment is but the vector addition of the private bond dipole moments. Being vectors, these can reinforce or cancel each other depending on the geometry of the molecule. Therefore, it is possible for molecules containing polar bonds to exist nonpolar overall, as in the example of carbon dioxide.

Molecular dipole moment of carbon dioxide: The linear shape of the CO2 molecule results in the canceling of the dipole moments of the two polar C=O bonds. The net, molecular dipole moment of CO2 is therefore zero, and the molecule is nonpolar.
H2O, by contrast, has a very large molecular dipole moment which results from the two polar H–O bonds forming an bending of 104.5° between them. The water molecule, therefore, is polar.
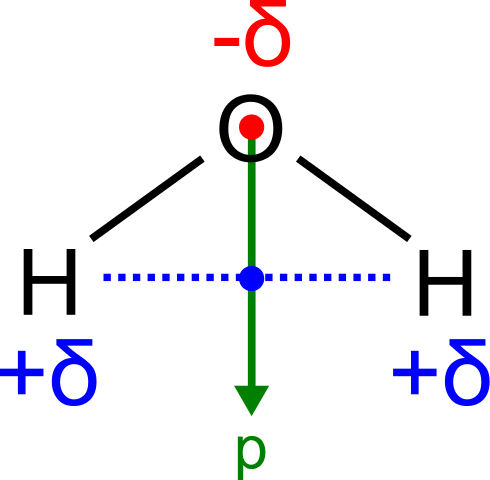
Dipole moment of a h2o molecule: Water has a very large dipole moment which results from the two polar H–O bonds oriented at an angle of 104.5° with respect to each other. The bond dipoles add upward to create a molecular dipole (indicated by the green pointer).
How Do You Think The Size Of An Atom Will Affect Its Ability To Attract Additional Electrons?,
Source: https://courses.lumenlearning.com/trident-boundless-chemistry/chapter/electronegativity/
Posted by: venturathereappos.blogspot.com


0 Response to "How Do You Think The Size Of An Atom Will Affect Its Ability To Attract Additional Electrons?"
Post a Comment